South Korea has implemented GHS for both substances and mixtures since 1 July 2013, which is based on 4th revision of UN GHS. All chemicals are now fully covered by GHS therefore the suppliers should ensure proper classification, and prepare and supply labels and Material Safety Data Sheets (MSDSs) to the downstream links.
Authorities and Regulations
There are three main ministries involved in GHS implementation, the Ministry of Employment and Labor (MoEL), the Ministry of Environment (MoE) and the National Emergency Management Agency (NEMA). Followings are the major regulations and standards.
Competent authorities | Regulations | Supporting standards | Note |
MoEL | Occupational Safety and Health Act (OSHA)
| Standard for Classification and Labeling of Chemical Substances and Material Safety Data Sheet (MoEL Public Notice No. 2016-19) | l Applicable to all chemicals meeting GHS hazard classification criteria; l Transitional periods: n substances till July 1, 2010; n mixtures till July 1, 2013
|
MoE | Chemicals Control Act (CCA); and Act on Registration, Evaluation, etc. of Chemicals (K-REACH)
| Regulation on Classification and Labelling of Chemical Substances | l Applicable to designated hazardous chemicals; l Transitional periods: n for existing substances: substances till July 1, 2011; mixtures till July 1, 2013; n New substances till July 1, 2008 |
NEMA | Act on Safety Control of Hazardous Materials (Old: Fire Service Act) | Standard for Classification and Labelling of Hazardous Materials | l Applicable to chemicals with flammability or ignitability l From November 13, 2008 |
Obligation
The Standard for Classification and Labeling of Chemical Substances and Material Safety Data Sheet (hereafter after referred to as the Standard) is the most important GHS standard in South Korea. It lays out the classification criteria for chemicals, methods of preparing and transferring of MSDS and label and the formats of them.
Classification
The following table show the building blocks adopted in South Korea. The building blocks highlighted in red are not covered by Korea GHS.

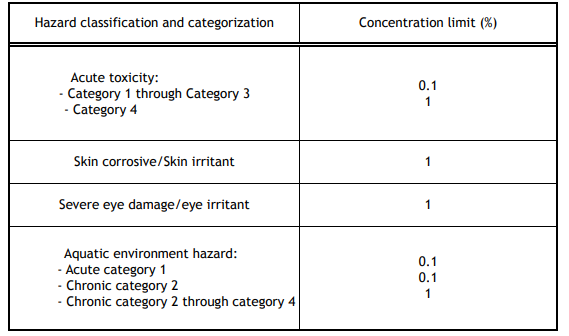
The Standard sets out the minimum concentrations for a substance which trigger the classification of a mixture if exceeded by the individual concentration or the sum of concentrations of relevant substances. Below are the hazard classes and categories where the generic concentration limits apply.
MSDS Requirements
South Korea’s MSDS consists of 16 sections. As a general rule, it shall be prepared in Korean. As for mixtures, when indicating the content of components in section 3, concentration ranges within ±5% (Lower value ~ upper value) is permissible. In this case, if the content is less than 5%, the lower value must be greater than 1% (special case: 0.1% for carcinogens and germ cell mutagens, 0.2% for respiratory sensitizers (limited to gases), and 0.3% for reproduction toxicants).
Label Requirements
Labels in Korean shall be indicated or printed on the containers and packaging of the chemicals to ensure that hazard and risk information is clearly labeled. For arrangement of labelling elements, the below key points should be considered.
Product Identifier: identical to that on the MSDS;
Signal word: If both “Danger” and “Warning” are applicable, only “Danger” shall appear;
Pictograms: If there are 5 or more pictograms, only 4 can appear;
Hazard statements (H statements): All hazard statements resulting from the classification must appear on the label, unless there is evident duplication or redundancy;
Precautionary statements (P statements): If the number of applicable precautionary statements is no less than 7, it is allowed to show only 6 of them on the label, with at least one precautionary statement selected from each subsection, namely prevention, response, storage and disposal (except when there is none applicable). In this case, it should be indicated on the label that you should refer to the MSDS for full precautionary statements.;
Supplier identification: Name, address and telephone number of the supplier in South Korea;
Simplified label for<100g or 100ml: H&P statements can be omitted; for semi-finished products used in the workplace: signal words only.
Example:

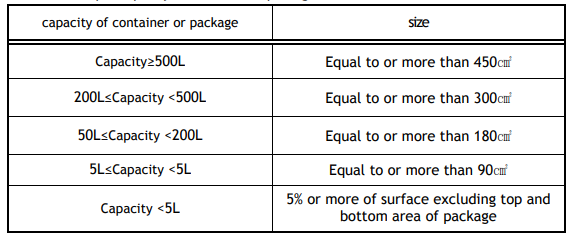
The minimum label size per capacity of containers or packages are specified as below. In addition, the size of a pictogram shall be at least 1/40 of the label and the minimum size shall not be less than 0.5 cm2.
Authority’s Lists
It is mandatory to follow MoE’s GHS Classification List in preparation of MSDS and label of designated substances. The list is dynamically updated by the Korea National Instituted of Environmental Research (NIER) to incorporate more hazardous substances and provide corresponding classification information, pictograms, signal words, hazard statements and M factor. So far, it contains 1,000 toxic substances, 13 types of restricted substances, 60 types of prohibited substances and 97 precautionary chemicals.
In addition, the Korean Occupational Safety and Health Agency (KOSHA) under MoEL has published classification, labelling information and MSDS samples for over 6,000 chemical substances on its homepage (http://msds.kosha.or.kr/kcic/english/msdssearch.do). These classifications are for reference only and are not mandatory. Self-classification by industry in accordance with OSHA classification criteria is allowed.
New MSDS Rules under OSHA (effective from January 1, 2021)
On January 15, 2019, the MoEL introduced an overhaul of OSHA (ChemLinked news). The most important changes made include the new requirements concerning SDS submission to MOEL, CBI application as well as the concept of “only representative” (OR), etc. Later, the OSHA Enforcement Decree and Enforcement Rule were revised in December 2019 accordingly (ChemLinked news). However, the revision to the most important Standard for Classification and Labeling of Chemical Substances and Material Safety Data Sheet hasn’t been finalized (ChemLinked news). Stakeholders need to keep a close eye on any updates in this space.
According to these new requirements, manufacturers or importers of chemical substances or mixtures which contain hazardous factors need to prepare and submit MSDS to the MoEL.
In the future you may choose between the following three choices:
(1) 100% composition disclosure on MSDS,
(2) Submit a MSDS indicating only hazardous factors and an additional document indicating information of other components,
(3) A MSDS indicating only hazardous factors and a letter of confirmation stating that there is no components falling under the hazards classification criteria.
A unique code will be assigned upon submission, which should be indicated on the top right corner of the MSDS for effective identification of products and information communication.
To address CBI concerns, the authorities allow stakeholders to apply for confidentiality protection of the chemical components with alternative name and content range. It is noted that harmful chemical substances that may cause serious health hazards to workers and the environment are not eligible for CBI claims. The CBI claim will be valid for three years and an extension application is required before expiration. It should be noted that the alternative name used on SDS for confidentiality protection should indicate the properties of the substance and potential hazards. A guidance will be fleshed out by MoEL to guide how to apply for CBI protection.
Overseas manufacturers are allowed to appoint an OR based in Korea to submit MSDS and apply for CBI protection. The appointment and dismissal of the OR shall be reported to the MoEL.
The provisions mentioned above regarding MSDS submission and CBI application will come into force on January 16, 2021. The new requirements will impose considerable new compliance burdens on the industry. To buffer industry transitional measures are introduced based on volume, including:
>1,000 t/y: before January 16, 2022
100-1,000 t/y: before January 16, 2023
10-100 t/y: before January 16, 2024
1-10 t/y: before January 16, 2025
<1 t/y: before January 16, 2026






